
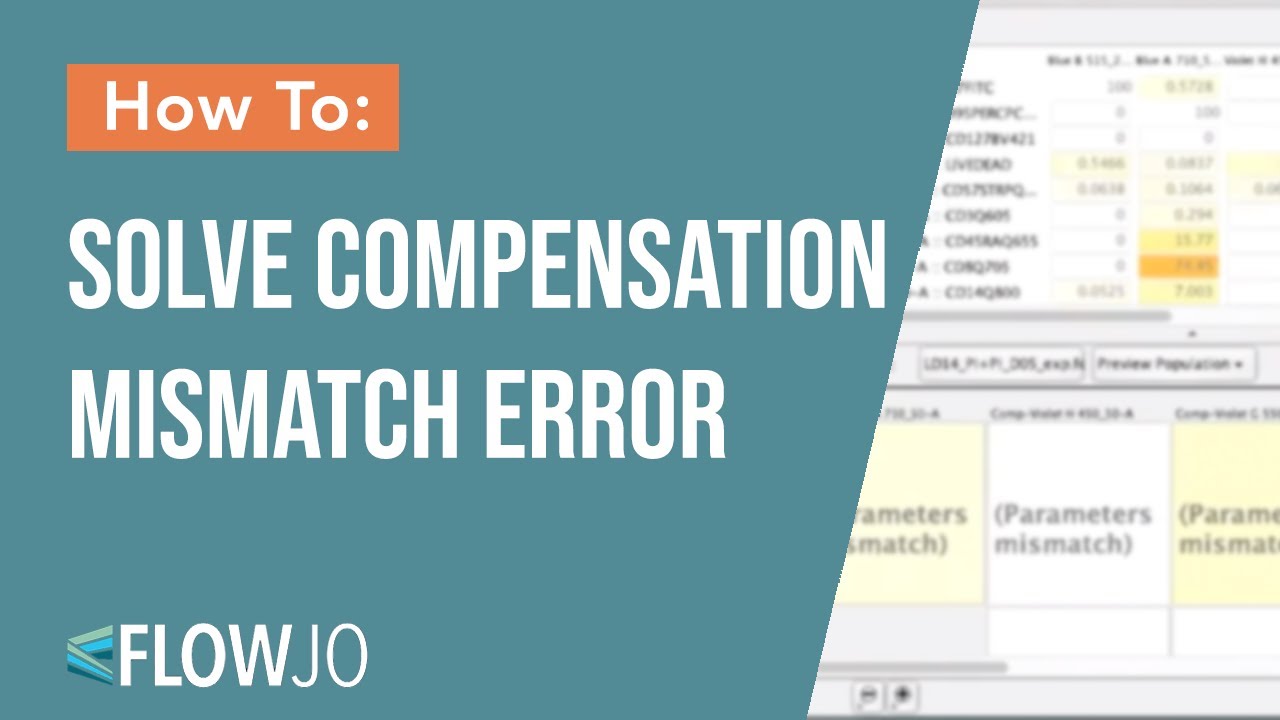
Are the parameters in the Compensation Wizard correct? Users should now be very careful to check the following three items:ġ.

Then, peak-finding algorithms will try to auto-gate negative and positive control populations within those samples. Opening the Compensation Wizard.Īt this point, FlowJo will perform some pretty cool magic tricks: First, it will attempt to automatically assign control samples to their appropriate colors. Click one of the two Compensation icons to open the Compensation Wizard:įigure 1.Load your single-stained controls into FlowJo, and make sure they are all added to FlowJo’s Compensation group.
#Compensation in flowjo professional#
This brief guide is to help users new to compensation calculations and experienced flow-maestros alike breeze through this process in a painless and professional fashion. Being a relatively new CyTOF user, I recently conjugated and titrated almost all my antibodies on helios.The Compensation Wizard in FlowJo is one of the most frequently used platforms, and by extension potentially the greatest source of confusion on a per-cytometrist basis.
#Compensation in flowjo how to#
So my question: how should I deal with this spillover? Specifically, how do you generate a proper compensation matrix for CyTOF data? I've tried to explain it with some figures (see the PDF in the link below, sorry I didn't figure out how to easily insert pictures into this post) In order to determine the concentration of antibody to use, I have been looking at the separation of signal (low vs high) and the amount of spillover the antibodies generate.Ĭoncerning this last point, I'm not really convinced that compensation is not required for CyTOF. I did this to the best of my abilities, but because I'm generating a panel to study mammary tissue, I don't have (m)any ab's that are exclusive.Īll comments and help are much appreciated! I asked around about this and (if people even bother with spillover) they tell me to properly titrate and swap metals around so you don't end up with weak ab's next to strong ab's and also you should know which ab's are mutually exclusive (so you know a certain cell can never have both antibody A and antibody B on it and if you do see it, you gate it out). I've tried applying compensation matrices to CyTOF data to deal with spillover, but for reasons I don't entirely understand it never seems to work particularly well. In the case of isotopic impurity in particular, which should be fixed, I would imagine that you should be able to apply a standard matrix based on the known isotopic ratios, but when I've tried to do this the resulting increase in negative noise seems to outweigh any benefits in reducing spillover (much like what you experienced). I believe the issue is related to the fact that even if the spillover in channel B may be a fixed percentage of channel A, at lower values of A it falls below the limit of detection in B. This means that instead of increasing linearly as signal A goes up, there is a lower/intermediate range of channel A where these is no apparent spillover into channel B and then an uptick once you hit the limit of detection (sort of a "hockey stick").


 0 kommentar(er)
0 kommentar(er)
